The ultimate goal in developing new synthetic methodologies in organic chemistry is to create molecular complexity from simple organic fragments in a minimum number of steps. Transition metal catalysis is one very powerful technique allowing difficult transformations by lowering the energy barrier and controlling the chemoselectivity and the stereochemical outcome of the reaction. Alkynes are very reactive and easily accessible organic functional groups which can be functionalized by various transition metal catalyzed transformations such as pericyclic reactions, carbonylation, hydroarylation, hydroamination, and many others. However, due to their very weak polarization, the functionalization of simple alkynes is difficult to control with a high level of regio- and stereoselectivity. With the project HydroMet, we would like to investigate further the transition metal-catalyzed hydrometallation of polarized triple C≡C bonds substituted by heteroatoms or functional groups and develop efficient methodologies to make this reaction regio- and stereoselective.
This project is funded by the Agence Nationale de la Recherche (ANR-18-CE07-0020)

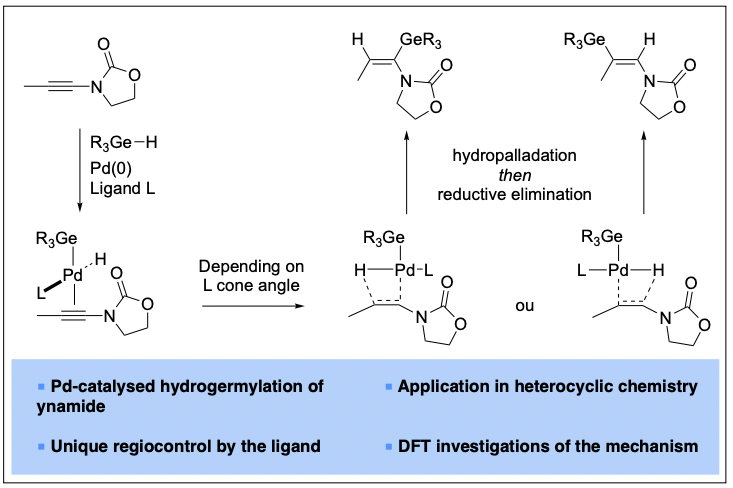
1) Ligand-Controlled Regiodivergent Palladium-Catalyzed Hydrogermylation of Ynamides
With complementary reactivities under radical, ionic, and metal-catalyzed conditions, ynamides are fascinating small molecules. We developed synthetic and DFT investigations of palladium-catalyzed ligand-controlled regiodivergent hydrometalation reactions of ynamides. Germylated and stannylated enamides are obtained with excellent α,E– or β,E-selectivities and a broad functional group tolerance. Such a regiodivergent palladium-catalyzed process is unique in ynamide chemistry and allows for the elaboration of metalated enamides that are useful building blocks for cross-coupling reactions or heterocyclic chemistry. In collaboration with Prof. K. N. Houk from the University of California (USA), DFT calculations fully support the experimental data and demonstrate the crucial roles of the trans-geometry of the [H-Pd(L)-Ge] complex, as well as of the steric requirements of the phosphine ligand. In addition, these calculations support the prevalence of a hydro-palladation pathway over a metal palladation of the π system of the ynamide.
J. Am. Chem. Soc. 2020, 142, 25, 11153–11164 (link)