2022-01- Synthesis and further use of SF5-alkynes as platforms for the design of more complex SF5-containing products
Popek, L.; Nguyen, T. M.; Blanchard, N.; Cahard, D.; Bizet V. Tetrahedron, 2022, 117-118, 132814.
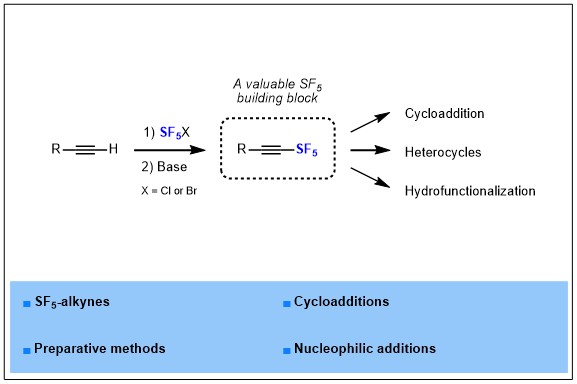
Pentafluorosulfanyl group is known as an emerging fluorinated group, it has been reported for the first time in 1960, and then remained an exotic fluorinated group for years, but nowadays this group is rising in interest and many groups and companies start to investigate this fluorinated motif in more depth. Indeed, SF5 is known as a “super CF3”, gathering and magnifying the benefits of CF3 that are a large volume, a high electronegativity and a high lipophilicity, which are highly valuable properties for drugs development. In parallel, a few applications of SF5-containing molecules has begun to appear in materials science, drug design or crop protection, but innovation remains highly dependent on the synthetic methods available to introduce SF5 in organic molecules. In this report, we turned our attention more specifically to SF5-alkynes, from their synthesis to applications.
2022-02- A common mechanism of Sec61 translocon inhibition by small molecules
Itskanov, S.; Wang, L.; Junne, T.; Sherriff, R.; Xiao, L.; Blanchard, N.; Shi, W. Q.; Forsyth, C.; Hoepfner, D.; Spiess, M.; Park, E. bioRxiv 2022, DOI:10.1101/2022.08.11.503542
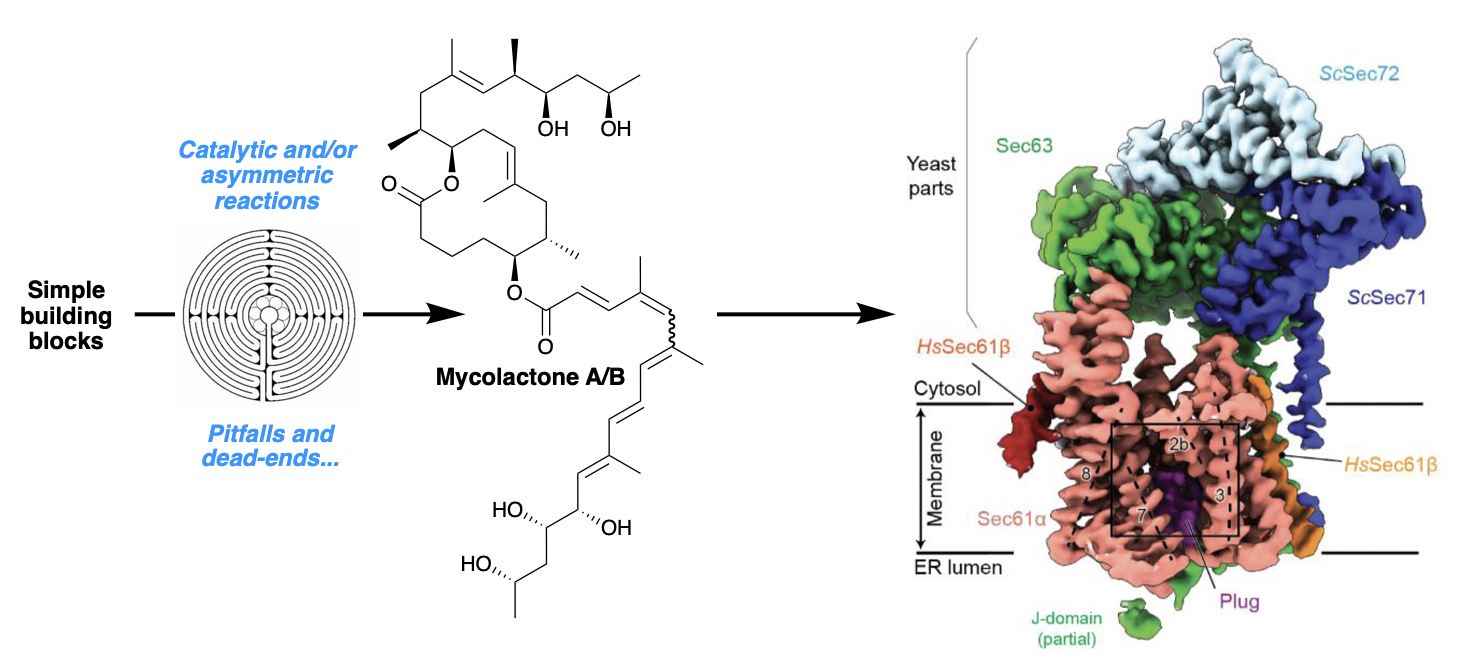
The Sec61 complex forms a protein-conducting channel in the endoplasmic reticulum (ER) membrane that is required for secretion of soluble proteins and production of many membrane proteins. Several natural and synthetic small molecules specifically inhibit the Sec61 channel, generating cellular effects that are potentially useful for therapeutic purposes, but their inhibitory mechanisms remain unclear. Here we present near-atomic-resolution structures of the human Sec61 channel inhibited by a comprehensive panel of structurally distinct small molecules— cotransin, decatransin, apratoxin F, ipomoeassin F, mycolactone, cyclotriazadisulfonamide (CADA) and eeyarestatin I (ESI). Remarkably, all inhibitors bind to a common lipid-exposed pocket formed by the partially open lateral gate and plug domain of the channel. Mutations conferring resistance to the inhibitors are clustered at this binding pocket. The structures indicate that Sec61 inhibitors stabilize the plug domain of Sec61 in a closed state, thereby preventing the protein-translocation pore from opening. Our study reveals molecular interactions between Sec61 and its inhibitors in atomic detail and offers the structural framework for further pharmacological studies and drug design.