2024-01- Palladium-Catalyzed Regioselective Synthesis of 2-SF5-Indenols and Further Derivatizations
Popek, L.; Cihan, M.; Blanchard, N.; Bizet V. Angew. Chem. Int. Ed., 2024, 63, e202315909.
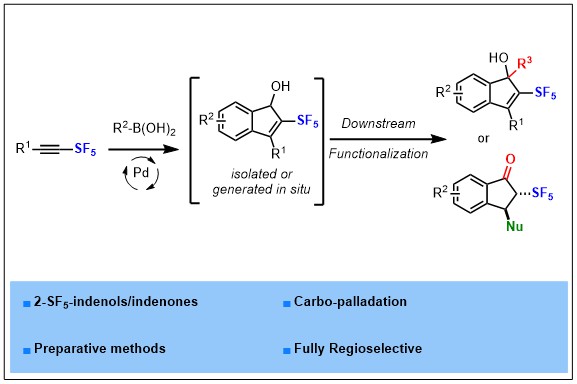
A palladium-catalyzed synthesis of 2-SF5-indenols has been developed by reacting commercially available boronic acid derivatives and readily accessible SF5-alkynes. The present methodology is fully regioselective thanks to the intrinsic polarization of SF5-alkynes. A selection of downstream functionalizations has been performed to highlight the versatility of 2-SF5-indenols and indenones as platforms for the design of more complex SF5-containing molecules.
2024-02- Expanding Radical Chloropentafluorosulfanylation of Alkynes
Nguyen, T. M.; Popek, L.; Matchavariani, D.; Blanchard, N.; Bizet, V.; Cahard, D. Org. Lett., 2024, 26, 365−369.
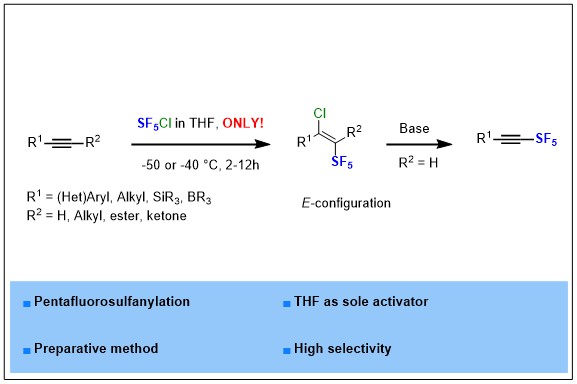
The chloropentafluorosulfanylation of alkynes is a delicate but crucial operation for accessing SF5-alkynes that serve as substrates in numerous transformations. Dolbier’s procedure using Et3B/O2 was the most efficient approach, while recent efforts make use of other initiators and light activation. We found that THF, as a single stimulus, is sufficient to trigger the reaction of SF5Cl with alkynes. We determined the configuration of Cl/SF5 products and clarified the structure of side-products.
2024-03- Ambiphilic Reactivity of SF5-Alkynes Applied to Regioselective and Stereodivergent Halogenation Reactions: An Experimental and Theoretical Case Study
Matchavariani, D.; Popek, L.; Cabrera Trujillo, J. J.; Nguyen, T. M.; Blanchard, N.; Miqueu, K.; Cahard, D.; Bizet, V.; Adv. Synth. Catal., 2024, 366, 3481-3493.
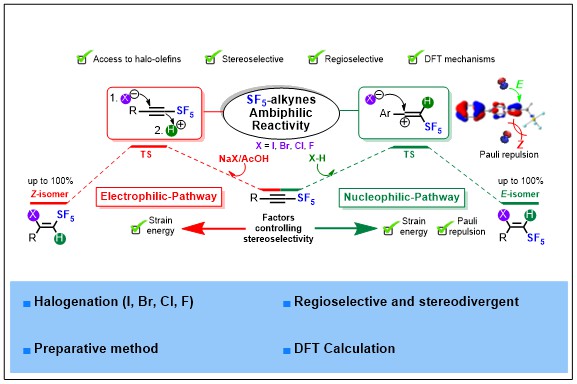
We explored the ambiphilic reactivity of SF5-alkynes, and we proved they can act as both nucleophiles and electrophiles. We selected halogenation reactions as benchmark reactions and developed highly selective stereodivergent hydrohalogenation (I, Br, Cl, F) reactions of SF5-alkynes. The stereochemistry is finely controlled thanks to the nature of the acids used (strong or soft) in the presence of halide source, while the high regioselectivity is governed by the strong polarization of SF5-alkynes. Mechanistic studies supported by DFT calculations shed light on two different reaction mechanisms responsible of the excellent stereocontrol. This stereoselectivity was quantitatively rationalized with the ASM and EDA methods. A few dihalogenation reactions are reported and DFT calculations rationalize this cis-stereoselectivity. Relative configuration of all the SF5-haloalkenes was unambiguously determined by X-ray diffraction. Noteworthy, several post-functionalization reactions such as cross-couplings, cyanation and reductions are described to strengthen the synthetic potential.
2024-04- Radical Reactions of Ynamides
Yang, Q.; Singh, D.; Debrauwer, V.; Bizet, V.; Blanchard, N., Chapter 5 in The Chemistry of Ynamides (Eds.: G. Evano, J. Zhao), Taylor & Francis, 2024, pp. 267-295.
Ynamides are versatile functional groups in modern organic chemistry, and they are used in a wide variety of transformations. However, only a few examples of radical reactions of ynamides are known, and their specific reactivity remains mainly underexplored. This chapter will first discuss intramolecular radical reactions of ynamides, which mainly deals with radical cyclizations leading to the straightforward synthesis of nitrogen heterocycles. Next, the intermolecular radical reactions will be overviewed, according to the nature of the C-X bond formed during the radical step. A last part will present oxidation reactions of ynamides following a specific reaction with molecular oxygen.

2024-05- Pericyclic Reactions of Ynamides
Rummler, L.; Brach, N.; Delmée, P.; Charton, L.; Bizet, V.; Blanchard, N. Chapter 9 in The Chemistry of Ynamides (Eds.: G. Evano, J. Zhao), Taylor & Francis, 2024, pp. 511-584.
Ynamides are versatile substrates in organic synthesis, the electron-rich character of the polarized ‘r-system makes them ideal substrates for regio-, chemo- and stereoselective transformations. Moreover, ynamides possess good affinity for Lewis and Brøinsted acids and they easily form highly reactive keteniminium ions upon reaction with an electrophile. Pericyclic reactions of ynamides have attracted a lot of interest from the synthetic community and are now well-established tools [1,2]. Depending on the substitution, the presence (and nature) of a catalyst and the reaction conditions, ynamides can be used as 2π- electrons partner (C≡C), 4π-electrons (for aromatic-substituted ynamides) or can even act as a three atoms system sharing π-electrons (N-C≡C). The present chapter, by no mean exhaustive, gathers pericyclic reactions of ynamides up to 2022 and is organized by classes of cycloaddition: [2+1] cycloadditions will be discussed first followed by [2+2], [3+2], [4+1], [4+2], [4+3], [5+1], [5+2] and, finally, [2+2+2] reactions.

2024-06- Copper-Catalyzed Regiodivergent Borylation of Fluorinated Alkynes and Palladium-Catalyzed Regiospecific Suzuki-Miyaura cross-coupling
Rummler, L.; Abd El Sater, M.; Blanchard, N.; Bizet, V. Synlett, 2024, 35, 1107-1112.
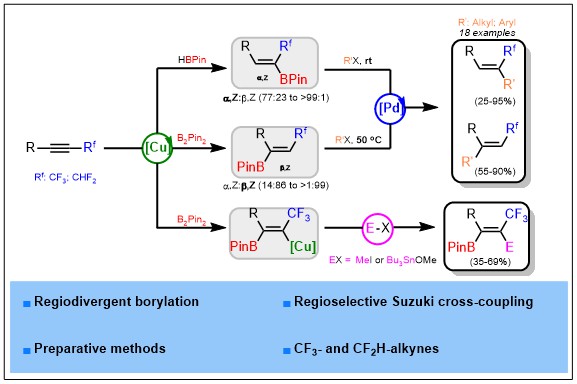
Alkenylboranes are key intermediates in modern organic synthesis, and they are usually obtained by borylation of alkynes. We report herein a highly regio- and stereoselective syntheses of α,Z- and β,Z-trifluoromethylated-alkenylboranes via copper-catalyzed borylation reactions. Further functionalization by palladium-catalyzed Suzuki-Miyaura cross-coupling have been developed and interestingly a drastic difference of reactivity between α- and β-borylated isomers has been identified.
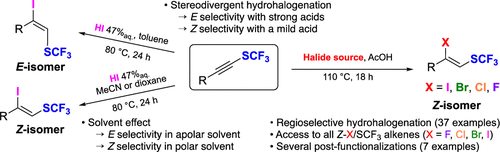
2024-07- Stereodivergent Hydrohalogenation of SCF3 Alkynes and Cross- Coupling Reactions
Marie, N.; Uyen Ly, K.; Shibata, N.; Bizet, V.; Cahard D. Org. Lett. 2024, 26, 9168-9172.
SCF3-substituted internal alkynes undergo regio- and stereoselective hydrohalogenation to provide the corresponding β-halo-α-SCF3-alkenes. The regioselectivity is ensured by the strong polarization of the C≡C triple bond in SCF3-alkynes, while the stereoselectivity is governed by the strength of the proton donor and/or by the solvent polarity. The potential of β-iodo-α-SCF3-alkenes was illustrated in several cross-coupling, protodeiodination, and cyanation reactions.
2024-08- Recent achievements in the synthesis and reactivity of pentafluorosulfanyl alkynes
René, F.; Clément–Comoy, L.; Blanchard, N.; Bizet, V. C. R. Chim. 2024, 27, 227-240.
In the dynamic field of pentafluorosulfanyl (SF5) chemistry, the SF5-alkynes have emerged as essential, readily accessible, and modular building blocks for the construction of a wide variety of SF5-containing molecules. This polarized platform has been used to perform highly regio-, chemo-, and stereoselective transformations such as heterocycle synthesis, cycloaddition, and hydroelementation reactions. This brief review provides an overview of recent developments in the synthesis and reactivity of SF5-alkynes, which are fascinating building blocks that have yet to reveal their full synthetic potential.

2024-09- Synthesis of Pentafluorosulfanylated Ynamides and Further Functionalizations
Popek, L.; Lefebvre, G.; Debrauwer, V.; Roubaud, D.; Blanchard, N.; Meyer, C.; Bizet, V. Org. Lett. 2024, 26, 10369–10375.
Herein is described the first synthesis of SF5-ynamides, versatile building blocks featuring the pentafluorosulfanyl motif. This synthesis proceeds through a two-step sequence of radical SF5-addition onto the π-system of a wide range of terminal ynamides and derivatives substituted with various nitrogen fragments followed by a dehydrochlorination reaction. A selection of downstream functionalization reactions, including nucleophilic additions, cycloadditions, and sulfur ylide synthesis, highlights the wide-ranging applications of this novel type of functionalized SF5-building block.
