2021-01- Synthesis and Physico-Chemical Properties of 2-SF5-(Aza)Indoles, A New Family of SF5-Heterocycles
Debrauwer, V.; Leito, I.; Lõkov, M.; Tshepelevitsh, S.; Parmentier, M.; Blanchard, N.; Bizet, V. ACS Org. Inorg. Au, 2021, 1, 43-50.
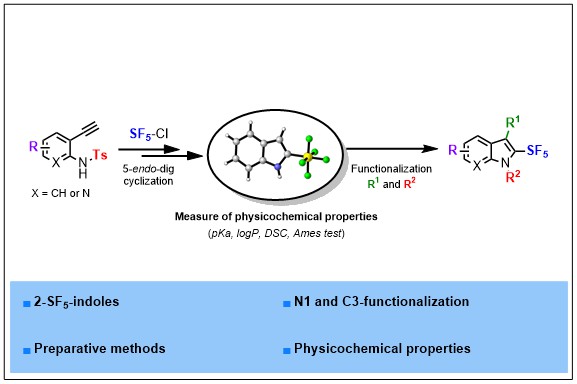
Structural diversity in heterocyclic chemistry is key to unlock new properties and modes of action. In this regard, heterocycles embedding emerging fluorinated substituents hold great promises. Herein is described a strategy to access 2-SF5-(aza)indoles for the first time. The sequence relies on the radical addition of SF5Cl to the alkynyl π-system of 2-ethynyl anilines followed by a cyclization reaction. A telescoped sequence is proposed making this strategy veryappealing and reproducible on gram scale. Downstream functionalizations are also demonstrated, allowing an easy diversification of N- and C3-positions. Ames test, pKa, logP and DSC measurements of several fluorinated 2-Rf-indoles are also disclosed. These studies highlight the strategic advantages that a C2- pentafluorosulfanylated motif impart to a privileged scaffold such as indole.
2021-02- Molecular Mechanisms Underpinning the Circulation and Cellular Uptake of Mycobacterium ulcerans Toxin Mycolactone
Tello Rubio, B.; Bugault, F.; Baudon, B.; Raynal, B.; Brûlé, S.; Morel, J.-D.; Saint-Auret, S.; Blanchard, N.; Demangel, C.; Guenin-Macé, L. Front. Pharmacol. 2021, 12, 733496.
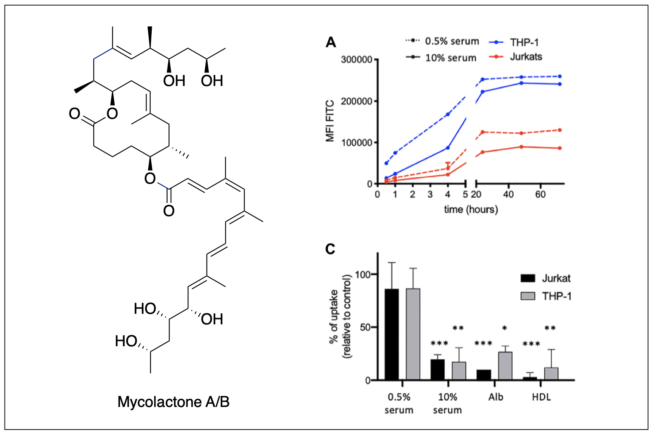
Mycolactone is a diffusible lipid toxin produced by Mycobacterium ulcerans, the causative agent of Buruli ulcer disease. Altough bacterially derived mycolactone has been shown to traffic from cutaneous foci of infection to the bloodstream, the mechanisms underpinning its access to systemic circulation and import by host cells remain largely unknown. We demonstrate that mycolactone specific association to serum albumin and lipoproteins is necessary for its solubilization and is a major mechanism to regulate its bioavailability. Overall, we suggest a new mechanism of transport and cell entry, challenging the dogma that the toxin enters host cells via passive diffusion.
2021-03- Oxetanes and Oxetenes: Fused-Ring Derivatives
Blanchard, N.; Bizet, V.; Brach, N.; Rummler, L.; Kaliappan, K. P. in Comprehensive Heterocyclic Chemistry IV, Vol. 2 (Eds.: D. S. Black, J. Cossy, C. V. Stevens), Elsevier, Oxford, 2022, pp. 257-286.
Fused and spirooxetanes, oxetenes and β-lactones are important classes of compounds that have found numerous applications in various areas of chemistry including medicinal chemistry and total synthesis of natural products. In this chapter, the main achievements from the theoretical and synthetic point of views since its last edition are discussed.
