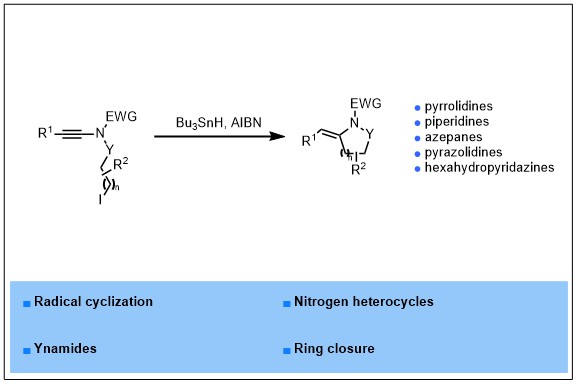
2023-01- Radical Cyclization of Ynamides to Nitrogen Heterocycles
Zhang, C.; Blanchard, N.; Evano G. Synthesis, 2023, 55, 272-288.
An efficient radical cyclization of suitably functionalized ynamides to nitrogen-containing heterocycles is reported. Upon reaction with tributyltin hydride in the presence of catalytic amounts of AIBN in toluene at 80 °C, a range of ynamides bearing a N-iodopropyl chain could be smoothly cyclized, in a highly regio- and stereo- selective manner, to the corresponding 2-arylidene-pyrrolidines in good to excellent yields. The exocyclic double bond was in addition shown to be an excellent anchor for further chemical diversification and the generality of this radical cyclization could be highlighted by its extension to the synthesis of other nitrogen heterocycles including piperidines, azepanes, pyrazolidines and hexahydropyridazines.
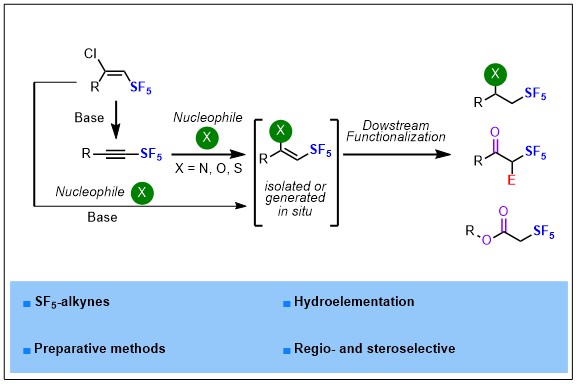
2023-02- Regio- and Stereoselective Hydroelementation of SF5-Alkynes and Further Functionalizations
Popek, L.; Cabrera-Trujillo, J. J.; Debrauwer, V.; Blanchard, N.; Miqueu, K.; Bizet, V. Angew. Chem. Int. Ed., 2023, 62, e202300685.
Herein is described a fully regio- and stereoselective hydroelementation reaction of SF5-alkynes with N, O and S-nucleophiles and further functionalization of the corresponding Z-(hetero)vinyl-SF5 intermediates, a suitable platform to access α-SF5 ketones and esters, β-SF5 amines and alcohols under mild reaction conditions. Experimental and computational comparative studies between SF5– and CF3-alkynes have been performed to highlight and explain the difference of reactivity and selectivity observed between these two fluorinated motifs.
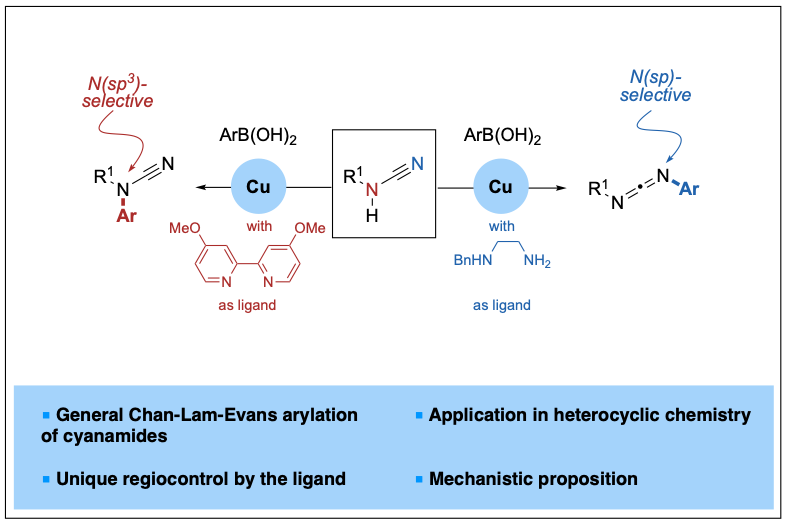
2023-03- Copper-Catalyzed, Ligand-Controlled N(sp3)- or N(sp)-Selective Arylation of Cyanamides
Fang, J.; Bekkouch, O.; Zeiser, G.; Zubchuk, Y.; Bizet, V.; Blanchard, N.; Evano, G. Org Lett. 2023, 25, 6446–6451.
Cyanamides possess both nucleophilic and electrophilic centers, and their arylation reactions are known to proceed at N(sp3) and C(sp) sites, leading to N-aryl cyanamides or amidines. N(sp) selectivity has also been reported only in the presence of amines, thus leading to guanidines. Herein, we report a general copper-catalyzed ligand-controlled Chan-Lam-Evans arylation of cyanamides proceeding regioselectively at the N(sp3) or N(sp) atoms and leading to either N-aryl cyanamides or dissymmetric carbodiimides. The nature of the ligand, either a bipyridine or a diamine, controls the product distribution and thus offers a divergent entry to useful building blocks from readily available cyanamides.
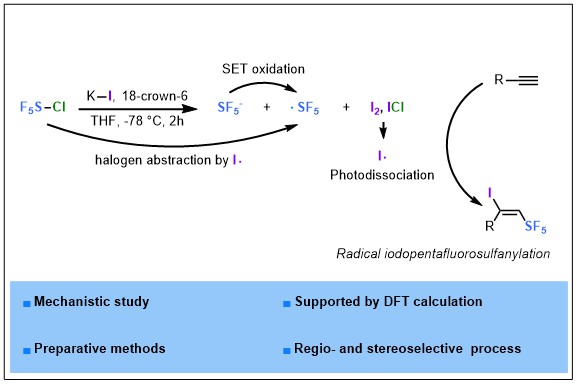
2023-04- Tracking SF5I in the Iodopentafluorosulfanylation of Alkynes
Nguyen, T. M.; Legault, C. Y.; Blanchard, N.; Bizet, V.; Cahard, D. Chem. Eur. J. 2023, 29, e202302914.
In the vibrant field of SF5 chemistry, SF5X reagents (X = F, Cl, Br) are at the heart of current investigations in radical pentafluorosulfanylation reactions. Alas! SF5I is the missing link whose existence has not been reported albeit its potential as SF5 donor. We herein report the formal addition of the hitherto unknown SF5I reagent to alkynes by means of a combination of SF5Cl / KI / 18-crown-6 ether. The exclusive regio- and stereoselective synthesis of unprecedented (E)-1-iodo-2-(pentafluoro-λ6-sulfanyl) alkenes was achieved. A consensus was reached through computational and mechanistic studies for the realistic formation of SF5– anion but not SF5I in solution and the rational involvement of SF5· and iodine radicals in the iodo pentafluorosulfanylation reaction.
2023-05- Fiche un point sur n° 108 – Le SF5 : un groupe fluoré à grand potentiel
Popek, L.; Matchavariani, D.; Bizet, V.; L’actualité chimique, N° 488, Octobre 2023.
Popular science article on the theme of SF5 chemistry, the challenges, synthetic methods and future potential application.
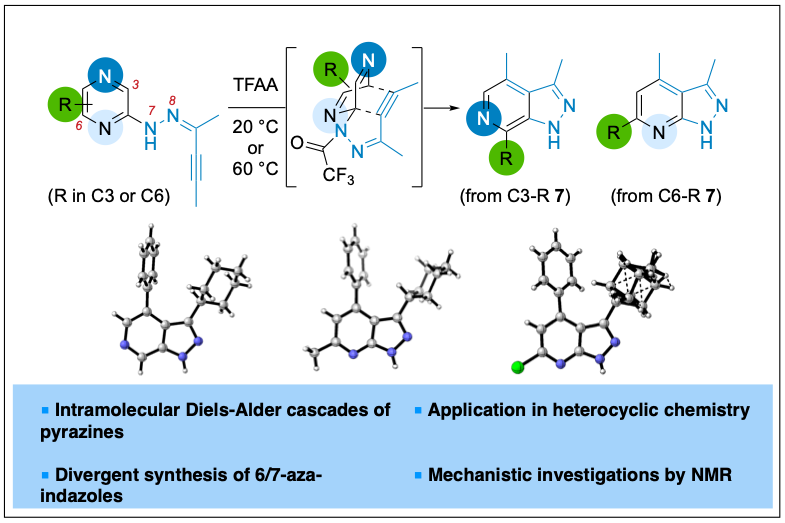
2023-07- Divergent Synthesis of 6- or 7-Aza-Indazoles via Intramolecular Diels–Alder Cascade of Pyrazines
Brach, N.; Popek, L.; Truong, M.; Laurent, C.; Bizet, V.; Kaliappan, K. P.; Blanchard, N. Org. Lett. 2023, 25, 7847-7851.
Highlighted in Synfacts 2024, 20, 128.
Pyrazines are reactive 4π partners in intermolecular Diels–Alder cycloaddition with exceptionally activated dienophiles or in an intermolecular version at elevated temperatures. Herein, it is shown that an intramolecular cascade could occur even at room temperature, delivering a collection of 6- or 7-aza-indazoles. An interesting substituent effect of the cycloaddition precursor on the product distribution was uncovered, and in situ NMR studies were conducted to gain insights into this unexpected selectivity.
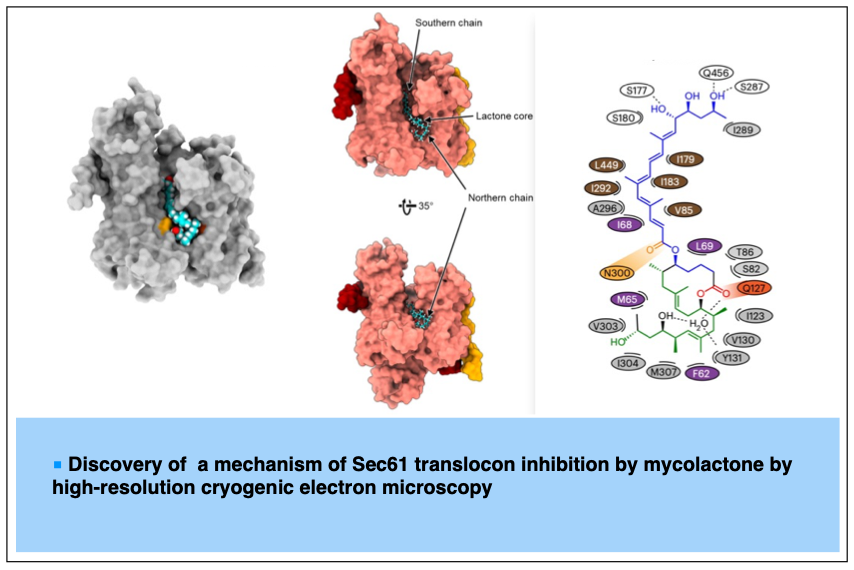
2023-08- A common mechanism of Sec61 translocon inhibition by small molecules
Itskanov, S.; Wang, L.; Junne, T.; Sherriff, R.; Xiao, L.; Blanchard, N.; Shi, W. Q.; Forsyth, C.; Hoepfner, D.; Spiess, M.; Park, E. Nature Chem. Biol. 2023, 19, 1063-1071.
The Sec61 complex forms a protein-conducting channel in the endoplasmic reticulum membrane that is required for secretion of soluble proteins and production of many membrane proteins. Several natural and synthetic small molecules specifically inhibit Sec61, generating cellular effects that are useful for therapeutic purposes, but their inhibitory mechanisms remain unclear. Here we present near-atomic-resolution structures of human Sec61 inhibited by a comprehensive panel of structurally distinct small molecules—cotransin, decatransin, apratoxin, ipomoeassin, mycolactone, cyclotriazadisulfonamide and eeyarestatin. All inhibitors bind to a common lipid-exposed pocket formed by the partially open lateral gate and plug domain of Sec61. Mutations conferring resistance to the inhibitors are clustered at this binding pocket. The structures indicate that Sec61 inhibitors stabilize the plug domain in a closed state, thereby preventing the protein-translocation pore from opening. Our study provides the atomic details of Sec61–inhibitor interactions and the structural framework for further pharmacological studies and drug design.