The objective of the DEFIS project is to developp efficient synthetic methods allowing access to a wide variety of compounds incorporating an emerging fluorinated group, with a special focus in our group on the pentafluorosulfanyl substituent. The interest of the new structures prepared will be demonstrated by their application in a wide range of chemical reactions which should lead to nitrogenous heterocycles substituted by the SF5 group and also allow to deepen the fundamental knowledge relating to the reactivity of organic compounds substituted with this emerging fluorinated group.
This project is funded by the Agence Nationale de la Recherche (ANR-17-CE07-0008)

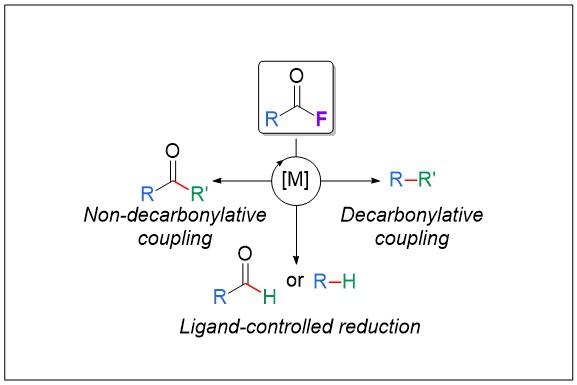
1) Acid Fluorides in Transition‐Metal Catalysis: A Good Balance between Stability and Reactivity
Several recent reports outlined the singular reactivity of acid fluorides as excellent electrophiles in transition‐metal catalysis. These species undergo oxidative addition of the metal into the C−F bond; then, retention or release of the CO moiety can occur and be controlled by tuning the catalytic system and the reaction parameters. Acid fluorides, which can be derived from carboxylic acids, show good stability and high reactivity in a wide range of possible functionalizations with nucleophiles. Their use provides an interesting alternative to that of the parent carboxylic acid derivatives (acid chlorides, esters, amides, acids, or aldehydes).
Angew. Chem. Int. Ed. 2019, 58, 6814-6817 (link)
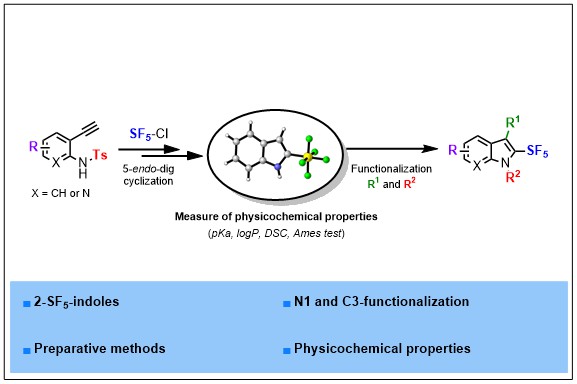
2) Synthesis and Physico-Chemical Properties of 2-SF5-(Aza)Indoles, A New Family of SF5-Heterocycles
Structural diversity in heterocyclic chemistry is key to unlock new properties and modes of action. In this regard, heterocycles embedding emerging fluorinated substituents hold great promises. Herein is described a strategy to access 2-SF5-(aza)indoles for the first time. The sequence relies on the radical addition of SF5Cl to the alkynyl Π-system of 2-ethynyl anilines followed by a cyclization reaction. A telescoped sequence is proposed making this strategy very appealing and reproducible on gram scale. Downstream functionalizations are also demonstrated, allowing an easy diversification of N- and C3-positions. Ames test, pKa, logP and DSC measurements of several fluorinated 2-Rf-indoles are also disclosed. These studies highlight the strategic advantages that a C2-pentafluorosulfanylated motif impart to a privileged scaffold such as indole.
ACS Org. Inorg. Au 2021, DOI:10.1021/acsorginorgau.1c00010 (link)