The pentafluorosulfanyl group is a truly new functional group introduced to organic chemists in the last decades. The paucity of hypervalent fluorinated sulfur-containing molecules is the result of unfavourable factors that are the poor availability of reagents for the introduction of the SF5 group, the lack of activation modes for SF5Cl or the notoriously inertness SF6, the absence of generalised methods to access λ6-pentafluorosulfanes, and thus a poor availability of SF5-containing building blocks, specifically aliphatic SF5 ones. We propose several levers of innovation to tackle the major bottlenecks that impede the bright future of SF5 chemistry. We hypothesize that new activation modes together with the design of safe reagents can be used to generate an extensive portfolio of novel SF5 compounds (WP1). Successful implementation of this fundamental research will create a sound foundation for the asymmetric construction of novel chiral non-racemic molecular architectures (WP2).
This project is funded by the Agence Nationale de la Recherche (ANR-21-CE07-0042)

1) Synthesis and further use of SF5-alkynes as platforms for the design of more complex SF5-containing products
Pentafluorosulfanyl group is known as an emerging fluorinated group, it has been reported for the first time in 1960, and then remained an exotic fluorinated group for years, but nowadays this group is rising in interest and many groups and companies start to investigate this fluorinated motif in more depth. Indeed, SF5 is known as a “super CF3”, gathering and magnifying the benefits of CF3 that are a large volume, a high electronegativity and a high lipophilicity, which are highly valuable properties for drugs development. In parallel, a few applications of SF5-containing molecules has begun to appear in materials science, drug design or crop protection, but innovation remains highly dependent on the synthetic methods available to introduce SF5 in organic molecules. In this report, we turned our attention more specifically to SF5-alkynes, from their synthesis to applications.
Tetrahedron, 2022, 117-118, 132814 (link)
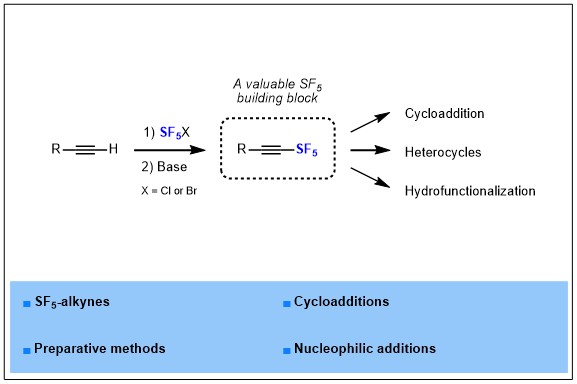
2) Tracking SF5I in the Iodopentafluorosulfanylation of Alkynes
In the vibrant field of SF5 chemistry, SF5X reagents (X = F, Cl, Br) are at the heart of current investigations in radical pentafluorosulfanylation reactions. Alas! SF5I is the missing link whose existence has not been reported albeit its potential as SF5 donor. We herein report the formal addition of the hitherto unknown SF5I reagent to alkynes by means of a combination of SF5Cl / KI / 18-crown-6 ether. The exclusive regio- and stereoselective synthesis of unprecedented (E)-1-iodo-2-(pentafluoro-λ6-sulfanyl) alkenes was achieved. A consensus was reached through computational and mechanistic studies for the realistic formation of SF5– anion but not SF5I in solution and the rational involvement of SF5· and iodine radicals in the iodo pentafluorosulfanylation reaction.
Chem. Eur. J. 2023, 29, e202302914 (link)
